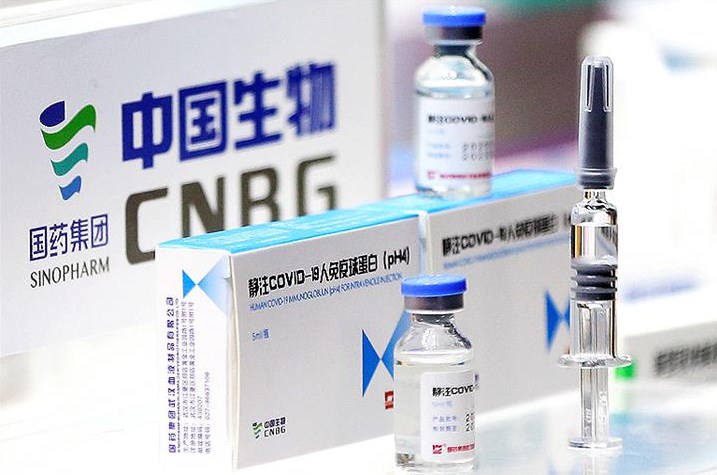
The World Health Organization (WHO) announced on Friday that China’s Sinopharm coronavirus vaccine had been granted emergency use authorization.
Sinopharm is already being used around the world, but the WHO‘s stamp of approval after a review of the safety and efficacy data could provide the public with a confidence boost in the jab.
It also opens the door to Sinopharm shipments being delivered through the WHO-backed COVAX initiative, an effort to give poorer countries access to approved vaccines.
“This expands the list of Covid-19 vaccines that COVAX can buy, and gives countries confidence to expedite their own regulatory approval, and to import and administer a vaccine,” WHO Director General Tedros Adhanom Ghebreyesus said at a news conference.
Sinopharm is the sixth vaccine to get WHO approval. It is administered in two doses for those 18 and older.
Source: dpa/MIA



Comments are closed for this post.