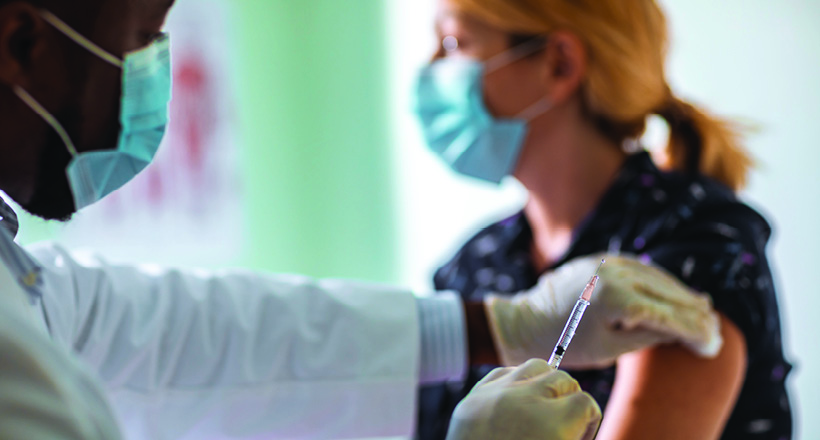
Johnson & Johnson’s coronavirus vaccine was paused in the United States and its roll-out in Europe and in South Africa was delayed, as health authorities investigate a potential link to rare blood clot events in a few recipients.
The worries compound the vaccine problems of the European Union, which lags far behind Britain, the US and Israel in getting shots in arms. Several EU countries are also in the midst of a third wave of cases that have sent citizens back under stricter lockdowns.
US federal health agencies made their recommendation on Tuesday after six blood clot cases were reported among the nearly 7 million people who have received the jab in the country.
All six cases of cerebral thrombosis involved women aged 18 to 48. The clots developed between six and 13 days after inoculation, the agencies said.
One woman died and a second woman was in critical condition. Three women developed thrombocytopenia, a lack of blood platelets.
The US Food and Drug Administration and the Centers for Disease Control described these adverse reactions as “extremely rare” but said use of the vaccine should be suspended while the data is examined, prompting many states to then announce the doses would be shelved.
The single-shot Johnson & Johnson vaccine has been used in the United States since last month and has been administered to more than 6.8 million people.
Jeff Zients, the White House Covid-19 coordinator, said the announcement would “not have a significant impact” on the US vaccination plan, noting the jab has made up less than 5 percent of all shots and that the country has secured enough doses from Pfizer/BioNTech and Moderna to be given to 300 million Americans.
Shortly after the shots were put on ice in the US, Johnson & Johnson announced that it would “proactively delay” the roll-out of the vaccine in Europe as investigations were under way.
The EU, which has struggled with vaccine supply shortages, started receiving its very first deliveries of Johnson & Johnson’s jab only on Monday, one month after it was given the green light for use in the bloc’s 27 member states.
Last week, the European Medicines Agency announced it was investigating four reported incidents of potentially deadly internal blood clots following vaccinations with Johnson & Johnson, without changing its recommendation status.
Earlier, EMA had identified a similar possible link between thrombosis and British-Swedish drugmaker AstraZeneca’s shot in very rare cases, but upheld its backing for the jab. The benefits very much outweighed risks, according to the EU regulator.
Still, many EU countries have limited the use of the AstraZeneca shot to people in older age groups.
South Africa also put the introduction of Johnson & Johnson’s coronavirus vaccine on hold due to the risk of possible blood clots in vaccinated people.
South Africa will suspend the roll-out of the vaccine over blood clot fears, Health Minister Zweli Mkhize said on Tuesday.
“We cannot take the decision of the FDA lightly. We’ve voluntarily suspend the roll-out until the causal relationship between the blood clot and the vaccine is sufficiently interrogated,” Mkhize told a media briefing on Tuesday night after the US decision, according to reports.
The vaccines from Johnson & Johnson and AstraZeneca are so-called vector-based vaccines. They use a harmless virus to introduce genetic information of the coronavirus into the body. This information helps spur the immune system to produce antibodies.
It is still unclear why the potentially harmful side effect occurs in some vaccinated people.
One possibility cited by Andreas Greinacher, a scientist from Germany’s Greifswald University that has studied the issue, is that the recipient produces a certain type of unusual antibody that leaves them susceptible to clotting.
The Moderna and Pfizer/BioNTech jabs work differently, sending a chemical mRNA messenger to teach cells how to trigger an immune response to the coronavirus.
Source: dpa/MIA



Comments are closed for this post.