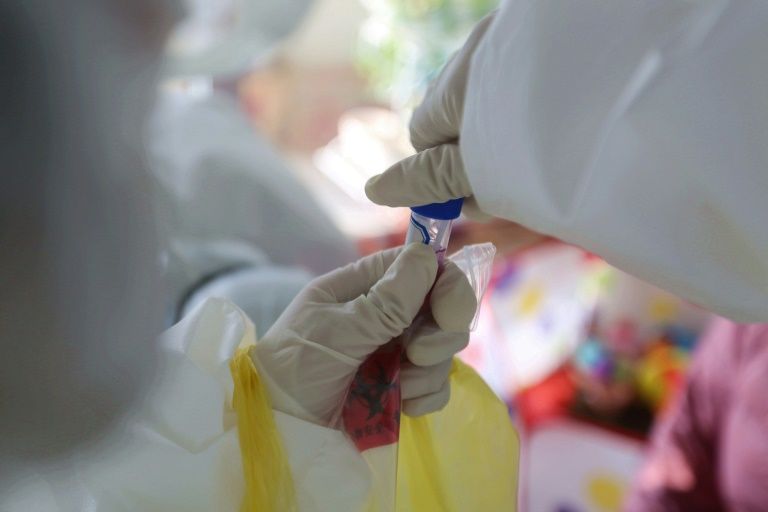
The first human volunteer has been dosed with a potential vaccine for the new coronavirus as a Phase 1 trial got under way in the US on Monday.
“This is the first of multiple steps in the clinical trial process for evaluating the potential benefit of the vaccine,” said the National Institutes of Health in a statement.
The Phase 1 trial is being done in Seattle and will involve 45 healthy adult volunteers ages 18 to 55 years, the NIH said.
However, Anthony Fauci, the head of the NIH, has repeatedly cautioned that it would take 12 to 18 months for a vaccine to be available for a public roll-out, to ensure safety and effectiveness.
Fauci said the current Phase 1 study was “launched in record speed.”
In large part, this was thanks to previous research on coronaviruses.
Chinese authorities shared the genetic sequence of the novel coronavirus in January. There is a race among different teams around the world, and in the US, to develop a potential vaccine.
The vaccine is called mRNA-1273 and is being developed with Moderna, a private sector biotech firm.
Moderna said it was “actively preparing” for Phase 2.
There are over 168,000 confirmed cases of Covid-19 and 6,610 deaths worldwide, according to the World Health Organization, with the numbers still rising as the virus spreads around the world and within countries.
There are no known specific treatments for the new virus, even as the vast majority of those who become infected get better.
Source: dpa/MIA



Comments are closed for this post.